The UK Government has legally-binding emission reduction commitments to reduce the amount of 5 key air pollutants and has committed to improving air quality in the UK by reducing emissions of these pollutants. These pollutants are outlined below.
Nitrogen Oxides (NOx)
Nitrogen oxides (NOx) are a group of gases that are formed during combustion of any fuel with oxygen at high temperatures. The majority of NOx emitted as a result of combustion is in the form of nitric oxide (NO). When NO reacts with other gases present in the air, it can form nitrogen dioxide (NO2) which is harmful to human health.
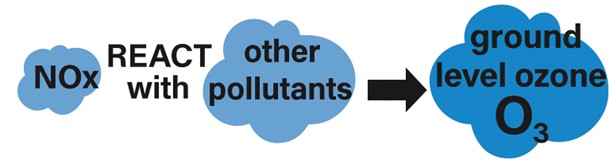
Image source: Clean Air Strategy 2019
It is also important in the formation of ozone (O3), which can impact both health and the environment. Ozone poses risks to health by triggering inflammation and asthma, but also causes damage to vegetation, including crops.
NO converts to NO2 quickly and this reaction is reversible; therefore, it is usual scientific practice to refer to the two gases together as NOx. For reporting and measurement purposes, NOx is reported as NO2 because of this fast interconversion.
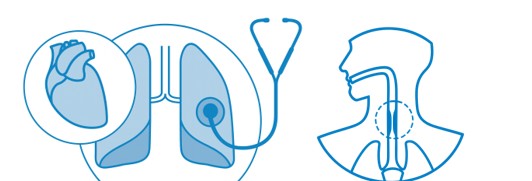
Image source: Clean Air Strategy 2019
Short-term exposure to concentrations of NO2 can cause inflammation of the airways and increase susceptibility to respiratory infections and to allergens. It exacerbates the symptoms of those who are already suffering from lung or heart conditions, shortening their lives.

Image source: Clean Air Strategy 2019
NOx can have a significant environmental impact. Nitrogen deposition, both as a gas (dry deposition) and through precipitation (wet deposition), can alter soil chemistry and affect biodiversity in sensitive habitat.
Sulphur Dioxide (SO2)
Sulphur dioxide (SO2) is a corrosive, acidic gas formed from the combustion of solid and liquid fuels that contain sulphur. It is harmful to health and combines with water vapour in the atmosphere to produce acid rain.
Image source: Clean Air Strategy 2019
SO2 pollution episodes are associated with asthma and chronic bronchitis and can also be a significant contributor to particulate matter in the air. Sulphur in coal played a key role in contributing to the health impacts of the London smog in 1952, where estimates of the resulting mortality range between 8,000 and 12,000 deaths.
Image source: Clean Air Strategy 2019
SO2 emissions caused significant harm to forests and freshwater habitats in the Northern Hemisphere in the 1970s – 1980s. Following concerted action to reduce SO2 emissions, such episodes no longer occur in the UK.
Emissions of SO2 have reduced considerably at the national level due to restrictions on the sulphur content of liquid fuels, alongside the shift away from reliance on coal for energy generation.
Particulate Matter (PM)
Particulate matter (PM) are solid particles or liquid droplets found in the air. It can come from natural sources such as pollen, sea spray and desert dust, as well as human made sources such as fires, vehicle exhausts, tyres and brakes. Particles emitted directly from these sources are called primary PM. Secondary PM is formed in the atmosphere through chemical reactions between other air pollutant gases such as nitrogen oxides (NOx), ammonia (NH3) and sulphur dioxide (SO2). Particulates are classified according to size, either as PM10 which are particles that are less than or equal to 10 micrometres in diameter, or PM2.5 which are particles that are less than or equal to 2.5 micrometres in diameter. PM2.5 particles are 200 times smaller than a grain of sand.
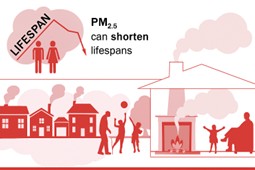
Image source: Clean Air Strategy 2019
PM is not a single pollutant; it is made up from a variety of chemical compounds and materials. Both PM, and the gases that can form it, can travel large distances and therefore impacts may occur far from the original source. Around 15% of UK PM comes from natural sources, up to a third from other European countries, and around half from UK human-made sources.
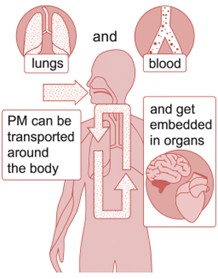
Image source: Clean Air Strategy 2019
Due to its size, PM can enter the lungs and bloodstream, allowing it to be transported around the body. PM can have short-term health impacts over a single day when concentrations are elevated, and long-term impacts from lower-level exposure over the course of someone’s life. Effects are amplified in vulnerable groups which include young children, the elderly, and those suffering from breathing problems like asthma.
Ammonia (NH3)
Ammonia (NH3) is a gas that is emitted into the atmosphere, from where it is either converted into secondary particulate matter (PM), or deposited back onto land. Agriculture is the dominant source of NH3 emissions and is emitted during storage and spreading of manures, slurries and fertilisers. After agriculture, the waste sector is the next most dominant source of NH3 emissions. Remaining NH3 emissions come from a mix of sources such as vehicles, human waste and industry.
Image source: Clean Air Strategy 2019
The main concerns resulting from NH3 emissions is its contribution to PM and the associated human health effects described under the PM section above. NH3 is converted into PM by mixing with nitrogen oxides (NOx) and sulphur dioxide (SO2), producing ammonium compounds that turn into fine PM. This PM is transported large distances and adds to the suspended background levels of particulates in the atmosphere. Public Health England attributed the 2014 London smog in part to agricultural NH3 emissions.
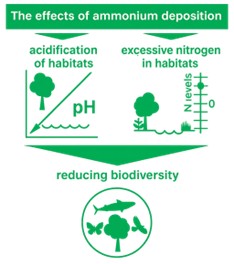
Image source: Clean Air Strategy 2019
NH3 stays in the atmosphere for just a few hours as a gas but this extends to several days when converted into PM. In this form it can travel long distances before being deposited on land by rain or snow. When deposited on land NH3 can cause significant impacts upon sensitive habitats, such as plants and wildlife.
Non-Methane Volatile Organic Compounds (NMVOCs)
Non-methane volatile organic compounds (NMVOCs) are a large group of organic compounds which differ widely in their chemical composition but can display similar behaviours in the atmosphere. NMVOCs are emitted into the air through combustion, such as domestic burning and transport, as well as from industrial processes, household products and agriculture.
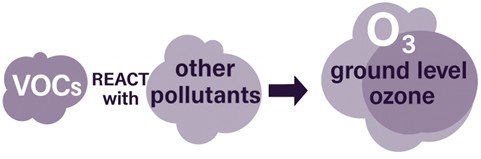
Image source: Clean Air Strategy 2019
Outdoors, NMVOCs react with other air pollutants in the presence of sunlight to produce ground level ozone (O3); however, NMVOCs emissions can also form a significant component of indoor air pollution.
Image source: Clean Air Strategy 2019
A particularly important NMVOC is formaldehyde which can be released from furniture, building materials and kitchen cabinets. Formaldehyde can also be formed by chemical reactions between other NMVOCs present in the air and chemicals generated from combustion processes, such as smoking, heating, cooking or candle burning. At low concentrations, exposure to formaldehyde can cause irritation to the eyes and upper airways and it is also classified as a human carcinogen.
Other sources of NMVOCs include furnishings, carpets, upholstery, products for cleaning and polishing, air fresheners, and personal care products, such as fragrances, deodorants, and hair styling products.

